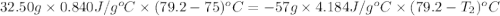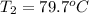Chemistry, 16.11.2019 02:31, JesusisLord2881
Apiece of glass with a mass of 32.50 g specific heat of 0.840 j/g*°c and an initial temperature of 75 °c was dropped into a calorimeter containing 57 g of water (specific heat 4.184 j/g*°c). the final temperature of the glass and water in the calorimeter was 79.2 °c. what was the initial temperature of the water?

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:00, sammiehammer
Use the table to identify the phase and phase changes of the elements under the given conditions. write the name of the substance, phase, or phase change. when the temperature in a room increases from 25°c to 33°c, changes from a solid to a liquid. in a lab, methane and nitrogen are cooled from -170°c to -200°c. the methane freezes and the nitrogen . when gold is heated to 2,856°c it changes from a liquid to a .
Answers: 2

Chemistry, 21.06.2019 23:30, mastershadow2018
Agroup of students is studying convection currents. they fill two identical balloons with the same amount of helium. one balloon is placed in a freezer and the other in an area with warm air. after 10 minutes, the balloons are released from a height of 1 meter. which of the following do the students most likely observe? a. the balloons both rise. the cold balloon is larger than the warm balloon. b. the balloons rise at the same rate. both balloons are the same size. c. the warm balloon expands and rises. the cold balloon shrinks and sinks. d. the cold balloon expands and rises. the warm balloon shrinks and sinks.
Answers: 2

Chemistry, 22.06.2019 14:30, darkghostmist
What type of reaction fuels the processes seen here?
Answers: 2
Do you know the correct answer?
Apiece of glass with a mass of 32.50 g specific heat of 0.840 j/g*°c and an initial temperature of 7...
Questions in other subjects:

Mathematics, 22.04.2020 20:36

Mathematics, 22.04.2020 20:36


Mathematics, 22.04.2020 20:36

Mathematics, 22.04.2020 20:36



Business, 22.04.2020 20:36


History, 22.04.2020 20:36


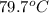
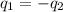
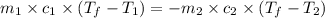
 = specific heat of glass =
= specific heat of glass = 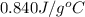
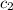 = specific heat of water =
= specific heat of water = 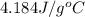
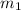 = mass of glass = 32.50 g
= mass of glass = 32.50 g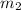 = mass of water = 57 g
= mass of water = 57 g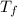 = final temperature of mixture =
= final temperature of mixture = 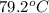
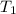 = initial temperature of glass =
= initial temperature of glass = 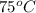
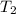 = initial temperature of water = ?
= initial temperature of water = ?