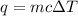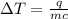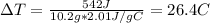The specific heat of a substance is 2.01 j/g·°
c. if given 10.3 grams of the substance and if...


Answers: 1
Similar questions

Chemistry, 01.07.2019 19:30, MaKynsi
Answers: 1

Chemistry, 07.07.2019 15:30, aliceohern
Answers: 1

Physics, 08.07.2019 08:00, stinkaq
Answers: 1

Chemistry, 31.07.2019 16:20, Maddy1212
Answers: 1
Do you know the correct answer?
Questions in other subjects:


Mathematics, 22.01.2021 05:50


Chemistry, 22.01.2021 05:50

Mathematics, 22.01.2021 05:50

Mathematics, 22.01.2021 05:50

Chemistry, 22.01.2021 05:50

Social Studies, 22.01.2021 05:50