
Chemistry, 18.09.2019 02:00, hchxxhBfncndnd9319
The haber process can be used to produce ammonia (nh3) from hydrogen gas (h2) and nitrogen gas (n2). the balanced equation for this process is shown below.
3h2 + n2 mc025-1.jpg 2nh3
the molar mass of nh3 is 17.03 g/mol. the molar mass of h2 is 2.0158 g/mol. in a particular reaction, 0.575 g of nh3 forms. what is the mass, in grams, of h2 that must have reacted, to the correct number of significant figures?
0.1
0.102
0.10209
0.1021

Answers: 2
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 21:00, lalaween098
What type of radiation is lead emitting in the following equation? alpha particles beta particles gamma rays
Answers: 3
Do you know the correct answer?
The haber process can be used to produce ammonia (nh3) from hydrogen gas (h2) and nitrogen gas (n2)....
Questions in other subjects:




Mathematics, 22.06.2019 22:20

Mathematics, 22.06.2019 22:20



English, 22.06.2019 22:20


Mathematics, 22.06.2019 22:20


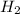 in grams is 0.102g.
in grams is 0.102g. = 17.03 g/mole
= 17.03 g/mole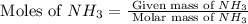 =
=  = 0.0337 moles
= 0.0337 moles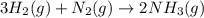
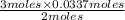 moles of
moles of 



