Answers: 1
Other questions on the subject: Chemistry


Chemistry, 21.06.2019 21:40, taysomoneyyy
During trial 2, what allowed you to determine that aluminum was the limiting reactant? check all that apply. all of the copper dissolved. all of the aluminum dissolved. the solution turned clear. the number of grams of copper(ii) chloride used in the reaction was greater than the number of grams of aluminum. the molar ratio of copper(ii) chloride to aluminum was greater than 3: 2, the equation’s molar ratio.
Answers: 2


Chemistry, 22.06.2019 11:40, arlabbe0606
Effect of rotenone and antimycin a on electron transfer rotenone, a toxic natural product from plants, strongly inhibits nadh dehydrogenase of insect and fish mitochondria. antimycin a, a toxic antibiotic, strongly inhibits the oxidation of ubiquinol. (a) explain why rotenone ingestion is lethal to some insect and fish species. (b) explain why antimycin a is a poison. (c) given that rotenone and antimycin a are equally effective in blocking their respective sites in the electron-transfer chain, which would be a more potent poison? explain.
Answers: 3
Do you know the correct answer?
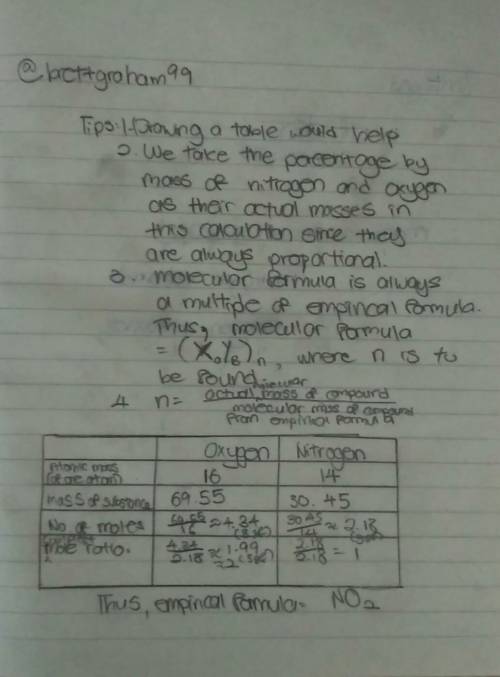
Find the molecular formula of a compound that contains 30.45% nitrogen and 69.55% oxygen. the molar...
Questions in other subjects:


English, 25.11.2021 07:30


Social Studies, 25.11.2021 07:30





Medicine, 25.11.2021 07:30

Chemistry, 25.11.2021 07:30