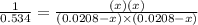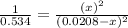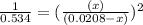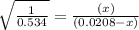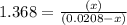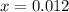Answers: 1
Other questions on the subject: Chemistry




Chemistry, 23.06.2019 09:00, hunterwilliams375
How many moles of potassium hydroxide are needed to completely react with 2.94 moles of aluminum sulfate according to the following equation:
Answers: 2
Do you know the correct answer?
For the reaction given below at 700°c, kc = 0.534. h2(g + co2(g h2o(g + co(g calculate the number of...
Questions in other subjects:

Chemistry, 13.03.2022 22:00



Mathematics, 13.03.2022 22:00

Mathematics, 13.03.2022 22:00




Mathematics, 13.03.2022 22:00


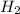 at equilibrium is 0.012 M
at equilibrium is 0.012 M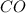 and
and 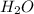 .
.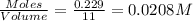
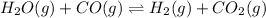
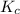 will be,
will be,![K_c=\frac{[H_2][CO_2]}{[H_2O][CO]}](/tpl/images/0263/7107/ded1c.png)
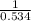 (for reverse reaction).
(for reverse reaction).