
Chemistry, 25.11.2019 08:31, triciazeeck62311
Deuterium is an isotope of hydrogen. the nucleus of a deuterium atom consists of one proton and one neutron. when two deuterium nuclei fuse, helium-3 is formed, and a neutron is emitted. which equation illustrates this process?
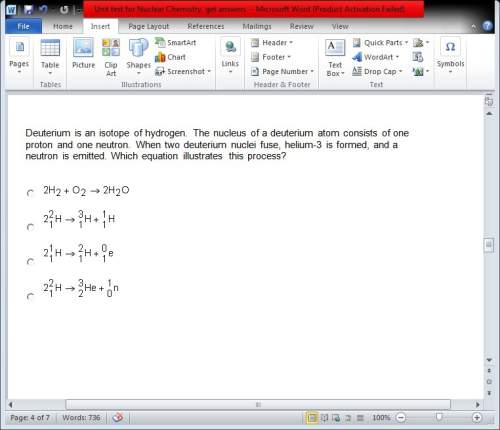

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:10, hunterthompson2
Which is true of transition metals when moving from left to right on the periodic table? the d sublevels are not filled across the period. the cation radii become larger across the period. atomic radii increase slightly and then start to decrease. atomic radii decrease slightly and then start to increase. o
Answers: 2

Chemistry, 22.06.2019 01:00, jescanarias22
Which statement correctly describes potassium iodide, ki? there is a one-to-one ratio of potassium ions to iodide ions. potassium gains electrons and iodine loses electrons during the reaction. the lattice is held together by potassium anions and iodide cations.
Answers: 1

Do you know the correct answer?
Deuterium is an isotope of hydrogen. the nucleus of a deuterium atom consists of one proton and one...
Questions in other subjects:


English, 21.05.2020 11:58



Social Studies, 21.05.2020 11:58


History, 21.05.2020 11:58








