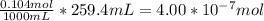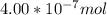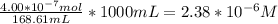Chemistry, 24.09.2019 12:00, divadebbgirl1
When a chemist titrates a standard solution of 168.61 ml of hydrochloric acid (hcl) with 0.104 m sodium hydroxide (naoh) , she finds that it requires 259.4 ml of the base to reach the endpoint of the titration. what is the molarity of the acid solution ?

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 23.06.2019 00:30, mariaramirez110379
On the periodic table, elements are arranged by which of the following. a. mass numbers. b. increasing atomic number. c. alphabetical order. or d. density
Answers: 1

Chemistry, 23.06.2019 01:30, Nakiahalogn4
How does the attraction between particles affect the ability of a solvent to dissolve in a substance
Answers: 1

Chemistry, 23.06.2019 03:30, elijahjacksonrp6z2o7
In general metals get as you move from left to right across the periodic table.
Answers: 1
Do you know the correct answer?
When a chemist titrates a standard solution of 168.61 ml of hydrochloric acid (hcl) with 0.104 m sod...
Questions in other subjects:

History, 24.07.2019 06:00

Computers and Technology, 24.07.2019 06:00

Computers and Technology, 24.07.2019 06:00

Computers and Technology, 24.07.2019 06:00

Mathematics, 24.07.2019 06:00

History, 24.07.2019 06:00

Chemistry, 24.07.2019 06:00

Social Studies, 24.07.2019 06:00

Chemistry, 24.07.2019 06:00