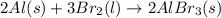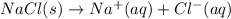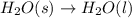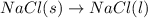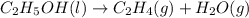Chemistry, 29.09.2019 05:30, rissacoob7862
Which of the following processes would you expect to have a negative value for entropy? nacl(s) na+(aq) + cl- (aq) h2o(s) h2o(l) nacl(s) nacl(l) 2 al(s) + 3br2(l) 2albr3(s) c2h5oh(l) c2h4(g) + h2o(g)

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:00, halohero7
3. how much energy in joules is required to evaporate .0005 kg of liquid ammonia to vapor at the same temperature? 4. how much energy ( in megajoules ) is given up by .75 kg of water at 0c when it freezes to form ice at 0c? 5. explain how heat works between and at critical temperatures?
Answers: 2

Chemistry, 22.06.2019 13:20, alejandra340
Can someone me with 3 and 4 plz. this is for masteries test.
Answers: 2

Chemistry, 23.06.2019 16:00, chrisraptorofficial
The table below shows a comparison of the different gas laws. some cells have been left blank. name variables constants equation boyle's law pressure, volume ? pv = k charles’s law volume, temperature ? v = kt ? temperature, pressure volume, moles of gas p = kt ? pressure, temperature, volume ? which are assumed to be constant while using the combined gas law? 1. pressure 2. number of moles 3. volume and moles of gas 4. pressure and temperature
Answers: 1

Chemistry, 23.06.2019 18:00, pleasedontspamme
Explain how compaction is important in the formation of coal.
Answers: 1
Do you know the correct answer?
Which of the following processes would you expect to have a negative value for entropy? nacl(s) na+...
Questions in other subjects:

Geography, 27.01.2020 18:31

Mathematics, 27.01.2020 18:31




Computers and Technology, 27.01.2020 18:31

Chemistry, 27.01.2020 18:31