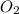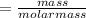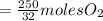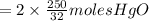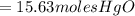Mercury(ii) oxide (hgo) decomposes to form mercury (hg) and oxygen (o2). the balanced chemical equation is shown below.
2hgo 2hg + o2
the molar mass of hgo is 216.59 g/mol. the molar mass of o2 is 32.00 g/mol. how many moles of hgo are needed to produce 250.0 g of o2?
3.906
7.813
15.63
73.87

Answers: 1
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 15:30, abdullaketbi71
What best discribes the relationship between wavelength and frequency in a electromagnetic wave
Answers: 1

Chemistry, 22.06.2019 17:40, Snowball080717
How much heat is added if 0.814g of water increase in temperature by 0.351 degree c?
Answers: 3
Do you know the correct answer?
Mercury(ii) oxide (hgo) decomposes to form mercury (hg) and oxygen (o2). the balanced chemical equat...
Questions in other subjects:

Health, 19.02.2021 23:20

History, 19.02.2021 23:20

Mathematics, 19.02.2021 23:20

Social Studies, 19.02.2021 23:20

Mathematics, 19.02.2021 23:20


Biology, 19.02.2021 23:20

Mathematics, 19.02.2021 23:20


Mathematics, 19.02.2021 23:20