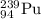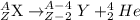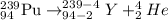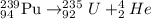Answers: 2
Other questions on the subject: Chemistry


Chemistry, 21.06.2019 23:00, carter1809
What is the molecular formula for a compound that is 46.16% carbon, 5.16% hydrogen, and 48.68% fluorine? the molar mass of the compound is 156.12 g/mol
Answers: 2

Chemistry, 22.06.2019 03:50, daniel9299
Consider the reaction: n2(g) + o2(g) ? 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3
Do you know the correct answer?
the alpha decay of a radioactive nuclide (x) emits a he-4 nucleus and produces an isotope of...
the alpha decay of a radioactive nuclide (x) emits a he-4 nucleus and produces an isotope of...
Questions in other subjects:

Biology, 22.10.2020 23:01

Mathematics, 22.10.2020 23:01

English, 22.10.2020 23:01


Mathematics, 22.10.2020 23:01


Mathematics, 22.10.2020 23:01

History, 22.10.2020 23:01

Chemistry, 22.10.2020 23:01

Mathematics, 22.10.2020 23:01