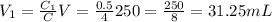Chemistry, 26.09.2019 11:10, stevensquad638
We have a solution of c5h10o2 of concentration 4 m. find out the volume we need to take from this solution to make a 250 ml solution of concentration 0.5 m.
is my result right? i got 31.25 ml
here's what i did:
m=moles solute / volume (l) solution (m=n/v)
first i calculate the moles of acid needed for a 0.5m, 0.25l solution.
n=m*v = 0.5*0.25 = 0.125 mol acid
now knowing how many moles i need to take, i calculate the volume those moles occupy in the 4m solution.
v=n/m = 0.125/4 = 0.03125 l solution = 31.25 ml
so you need to take 31.25 ml of 4m acid solution and add 218.75 (250*31.25) of water to make a 0.5m, 250 ml solution.

Answers: 1
Other questions on the subject: Chemistry


Do you know the correct answer?
We have a solution of c5h10o2 of concentration 4 m. find out the volume we need to take from this so...
Questions in other subjects:

Mathematics, 06.12.2020 14:00



English, 06.12.2020 14:00



Biology, 06.12.2020 14:00

Mathematics, 06.12.2020 14:00


Medicine, 06.12.2020 14:00


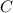 .
.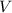 mL solution of concentration
mL solution of concentration 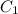 , and we want to know which volume
, and we want to know which volume 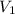 of solution to take from the original to do that.
of solution to take from the original to do that.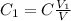 hence
hence