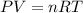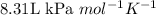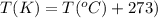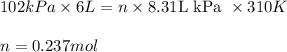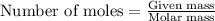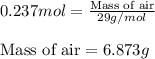Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:00, 2024daisjavien
Which actions would increase the rate at salt dissolves in water? stir the water? crush the salt? use less water? heat the water? cool the salt
Answers: 3

Chemistry, 22.06.2019 04:30, terrancebest
Which is a chemical property of iron? a. it forms iron oxide (rust) when exposed to moisture and air. b. it is a gray–black metal that is hard to the touch. c. it has a melting point of 2795°f (1536°c). d. it is a good conductor of heat
Answers: 2


Chemistry, 23.06.2019 03:00, draveon6925
Achemical equilibrium between gaseous reactants and products is shown. n2(g) + 3h2(g) ⇌ 2nh3(g) how will the reaction be affected if the pressure on the system is increased? it will shift toward the reactant side as there is lower pressure on the reactant side. it will shift toward the product side as there is higher pressure on the product side. it will shift toward the reactant side as there are a greater number of moles of gas on the reactant side. it will shift toward the product side as there are a fewer number of moles of gas on the product side.
Answers: 2
Do you know the correct answer?
An average adult has a total lung capacity of 6.0 l. how many total grams of air could be held in th...
Questions in other subjects:

Mathematics, 16.05.2021 09:50

Mathematics, 16.05.2021 09:50



Mathematics, 16.05.2021 09:50


Computers and Technology, 16.05.2021 09:50


Mathematics, 16.05.2021 14:00