
Chemistry, 03.11.2019 01:31, jjjjjjgegi3088
State and explain the effect of increasing the pressure on the yield of ammonia in this reaction (decomposition of ammonium carbonate).
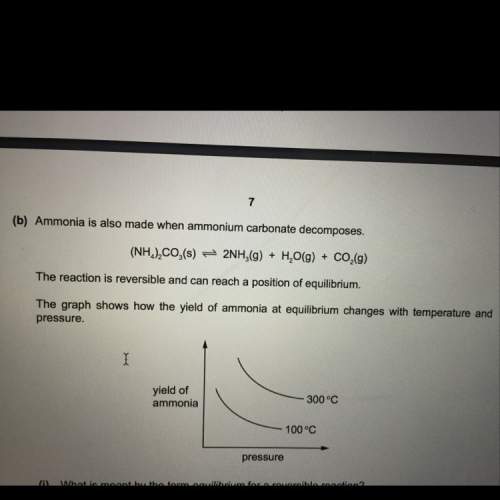

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:30, perezanthony2403
Which describes fat? a: a carbohydrate that produces energy b: a nucleic acid that directs cell function c: a lipid that stores energy d: a protein that speeds up a chemical reaction
Answers: 1

Chemistry, 22.06.2019 23:30, sanociahnoel
The density of the solid phase of a substance is 0.90 g/cm3 and the density of the liquid phase is 1.0 g/cm3. a large increase in pressure will a. lower the freezing point b. raise the freezing point c. lower the boiling point d. raise the triple point e. lower the triple point
Answers: 1

Chemistry, 23.06.2019 00:30, runninglovexoxo
The molecular weight of carbon dioxide, co2, is 44.00 amu, and the molecular weight of nitrous dioxide, no2, is 46.01 amu, so no2 diffuses co2
Answers: 2

Chemistry, 23.06.2019 16:00, michibabiee
Which of the following is a reason to make an armature the parent of a creature
Answers: 1
Do you know the correct answer?
State and explain the effect of increasing the pressure on the yield of ammonia in this reaction (de...
Questions in other subjects:

Advanced Placement (AP), 13.10.2020 18:01

Mathematics, 13.10.2020 18:01


Biology, 13.10.2020 18:01

Mathematics, 13.10.2020 18:01

History, 13.10.2020 18:01

Mathematics, 13.10.2020 18:01









