
Chemistry, 29.01.2020 08:00, kellynadine02
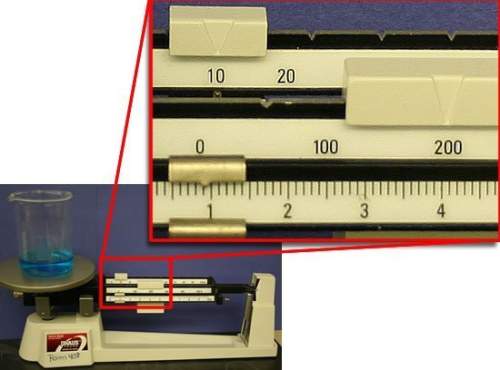
what is the mass of water in the beaker? assume that the beaker has a mass of exactly 100.00 g.
a) 100.90 g
b) 109.00 g
c) 110.90 g
d) 210.90 g

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 13:30, amandajbrewerdavis
Table sugar completely dissolved in water is an example of a?
Answers: 1

Chemistry, 22.06.2019 18:00, brisacruz013
Which statement best describes the he properties of iconic compounds ?
Answers: 1

Chemistry, 22.06.2019 21:30, emmalucilleblaha1995
Achemical reaction is done in the setup shown, resulting in a change of mass. what will happen if the same reaction is done in a sealed container that is placed on the electronic balance?
Answers: 1
Do you know the correct answer?
what is the mass of water in the beaker? assume that the beaker has a mass of exactly 100.00 g.
Questions in other subjects:

Physics, 11.01.2020 20:31



Chemistry, 11.01.2020 20:31

Chemistry, 11.01.2020 20:31

Biology, 11.01.2020 20:31


History, 11.01.2020 20:31


English, 11.01.2020 20:31






