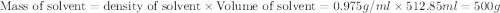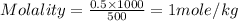Chemistry, 22.12.2019 21:31, amandasantiago2001
Given that the density of water is 0.975 g/ml and that 171 g of sucrose (molar mass: 342.30 g/mol) is dissolved in 512.85 ml of water at 80°c, what is the molality of this solution?

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 13:30, annanikherrera
If the concentration of phosphate in the cytosol is 2.0 mm and the concentration of phosphate in the surrounding fluid is 0.1 mm, how could the cell increase the concentration of phosphate in the cytosol? a) passive transportb) diffusionc) active transportd) osmosise) facilitated diffusion
Answers: 3

Chemistry, 22.06.2019 16:00, julesperez22
Click the button that shows the correct relationship of the electron affinities of the elements sodium and phosphorus. sodium’s electron affinity value is more negative than the electron affinity value of phosphorus. phosphorus’ electron affinity value is more negative than the electron affinity value of sodium. this information cannot be determined using the periodic table. answer is b on e2020.
Answers: 3
Do you know the correct answer?
Given that the density of water is 0.975 g/ml and that 171 g of sucrose (molar mass: 342.30 g/mol)...
Questions in other subjects:


Mathematics, 08.04.2021 22:40


Mathematics, 08.04.2021 22:40


Biology, 08.04.2021 22:40

Mathematics, 08.04.2021 22:40

Mathematics, 08.04.2021 22:40

Arts, 08.04.2021 22:40


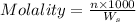
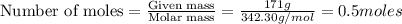
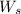 = weight of solvent in g
= weight of solvent in g