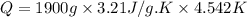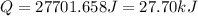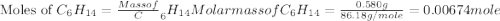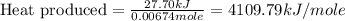Chemistry, 19.11.2019 04:31, billgray2571
Asample of hexane (c6h14) has a mass of 0.580 g. the sample is burned in a bomb calorimeter that has a mass of 1.900 kg and a specific heat of 3.21 j/gik. what amount of heat is produced during the combustion of hexane if the temperature of the calorimeter increases by 4.542 k?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:00, BaileyElizabethRay
Mitosis is a type of cell division that produces cells that are identical to the parent cell. meiosis is a different type of cell division that produces cells that carry have a genetic material of the parent cell. based on the information provided how do the purpose of mitosis and meiosis differ
Answers: 3

Chemistry, 22.06.2019 11:00, bigwaYne
Imagine that twenty i. u.’s of enzyme z were catalyzing the above reaction for one minute, under vmaxconditions, in a 3.00 ml assay volume. the assay is buffered with 20 mm phosphate buffer, ph 7.60. what will the ph be at the end of that one minute?
Answers: 2

Chemistry, 22.06.2019 14:40, sugardime
Choose an equation that represents an enzyme-catalyzed reaction. (a) enzyme + substrate → enzyme-substrate complex (b) enzyme + substrate ←−→ enzyme + products (c) enzyme + substrate ←−→ enzyme-substrate complex → enzyme + products (d) enzyme + substrate ←−→ enzyme-substrate complex → enzyme-substrate complex + products
Answers: 2

Chemistry, 22.06.2019 15:30, lizzyhearts
Count the number of each type of atom in the equation below, and then balance the equation. write in the numbers of atoms and coefficients. add a 1 if there should be no coefficient. cs2(l) + o2(g) → co2(g) + so2(g) c [ ] s [ ] o > c [ ] s [ ] o [ ] cs2(l) + [ ] o2(g) > [ ] co2(g) + [ ] so2(g)
Answers: 3
Do you know the correct answer?
Asample of hexane (c6h14) has a mass of 0.580 g. the sample is burned in a bomb calorimeter that has...
Questions in other subjects:

History, 17.11.2019 02:31

Mathematics, 17.11.2019 02:31


Mathematics, 17.11.2019 02:31



Mathematics, 17.11.2019 02:31

Health, 17.11.2019 02:31



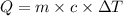
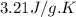
 = change in temperature = 4.542 K
= change in temperature = 4.542 K