Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:30, gwenparks
Calculate the ratio of h+ ions to oh– ions at a ph = 7. find the concentration of h+ ions to oh– ions listed in table b of your student guide. then divide the h+ concentration by the oh– concentration. record this calculated ratio in table a of your student guide. compare your approximated and calculated ratios of h+ ions to oh– ions at a ph = 7. are they the same? why or why not? record your comparison in table a. what is the concentration of h+ ions at a ph = 7? mol/l what is the concentration of oh– ions at a ph = 7? mol/l what is the ratio of h+ ions to oh– ions at a ph = 7? : 1
Answers: 1

Chemistry, 22.06.2019 14:30, emilymartinez75
Need ! asap will mark 10 pts using the room temperature line (orange line) and your periodic table, make lists that identify the state of matter (gas, liquid, or solid) in which each element you plotted exists at room temperature. explain your answers
Answers: 1


Chemistry, 23.06.2019 01:00, ZaNiyahlove4711
Which of the following is the molecular formula for a simple sugar? a. cooh b. h2o c. oh d. c6h12o6
Answers: 1
Do you know the correct answer?
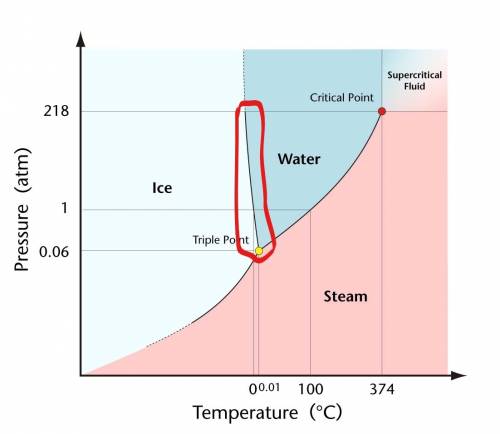
Under certain conditions, the solid and liquid states of water can exist in equilibrium. how are the...
Questions in other subjects:

Social Studies, 01.04.2021 02:50

Mathematics, 01.04.2021 02:50

Mathematics, 01.04.2021 02:50

Mathematics, 01.04.2021 02:50

Chemistry, 01.04.2021 02:50

French, 01.04.2021 02:50

Law, 01.04.2021 02:50


Mathematics, 01.04.2021 02:50