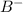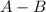Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 21:30, liamgreene90
In one or two grammatically correct sentences, write a definition for the term molecule geometry
Answers: 3

Chemistry, 22.06.2019 22:40, lindseyklewis1p56uvi
Percent ionization for a weak acid (ha) is determined by the following formula: percent ionization=[ha] ionized[ha] initial×100%for strong acids, ionization is nearly complete (100%) at most concentrations. however, for weak acids, the percent ionization changes significantly with concentration. the more diluted the acid is, the greater percent ionization. a certain weak acid, ha, has a ka value of 9.4×10? 7.part acalculate the percent ionization of ha in a 0.10 m solution. part bcalculate the percent ionization of ha in a 0.010 m solution
Answers: 1


Do you know the correct answer?
What is the definition of a lewis acid? a substance that accepts electrons to form a covalent bond...
Questions in other subjects:

Geography, 01.12.2021 18:00


English, 01.12.2021 18:00

English, 01.12.2021 18:00


Social Studies, 01.12.2021 18:00

History, 01.12.2021 18:00

Mathematics, 01.12.2021 18:00

Mathematics, 01.12.2021 18:00


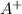 +
+