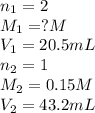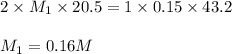Atitration was performed in a lab situation. h2so4 was titrated with naoh. the following data was collected: ml of naoh used = 43.2 ml concentration naoh = 0.15 m ml h2so4 = 20.5 ml notice that h2so4 releases 2 h+ per mole. what is the concentration of h2so4? 0.036 m 0.16 m 0.63 m 6.3 m

Answers: 2
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 03:10, emilyplays474
The peak wavelength for the blackbody curve of a star is in the uv range. assuming the radiation from this star can reach earth, would you be able to see it?
Answers: 2

Chemistry, 22.06.2019 09:00, alydiale584
Particles vibrate in a rigid structure and do not move relative to their neighbors.
Answers: 1
Do you know the correct answer?
Atitration was performed in a lab situation. h2so4 was titrated with naoh. the following data was co...
Questions in other subjects:



Mathematics, 15.09.2021 21:20

Mathematics, 15.09.2021 21:20

English, 15.09.2021 21:20


Mathematics, 15.09.2021 21:20

Health, 15.09.2021 21:20



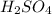 comes out to be 0.16 M.
comes out to be 0.16 M.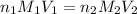
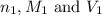 are the n-factor, molarity and volume of acid which is
are the n-factor, molarity and volume of acid which is 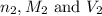 are the n-factor, molarity and volume of base which is NaOH.
are the n-factor, molarity and volume of base which is NaOH.