Chemistry, 31.01.2020 09:48, sierras0330
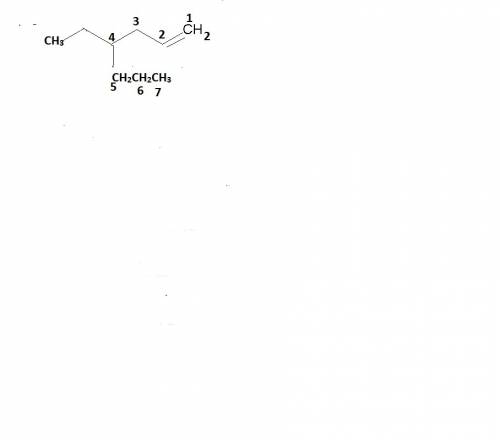
What is the best name for the molecule below? (2 points)
a compound with a total of nine carbons with a double bond and the rest single bonds. there is a straight chain of six carbons along the bottom with a double bond between the fifth and sixth carbons when counting from left to right. there is a propyl group branching off of the third carbon from the left.
4-ethyl-6-heptene
4-propyl-1-hexene
4-ethyl-1-heptene
3-propyl-5-hexene

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:00, pettygirl13
Describe the particles of all three phases of matter in the root beer float. (how are the particles arranged and moving? )
Answers: 3

Chemistry, 22.06.2019 00:30, bryneosburn
Lem 2 the data below are for the system ethyl propyl ether (1)-chloroform (2) at 0.5 bar. use the data to answer the following questions (all questions refer to p d 0: 5 bar). a) what are the boiling points of the pure components at 0.5 bar? b) a mixture with the overall composition z1 d 0: 1 is brought to 47.6ä±c, 0.5 bar. what is the phase? c) 100 mole of a mixture with z1 d 0: 1 (state a) is mixed with 22 mole of pure ethyl propyl ether vapor (state b). the mixing takes place at 47.6 ä±c, 0.5. bar. what is the phase of the resulting mixture (state c)? if the state is a v/l mixture report the number of moles and mole fractions in each phase. d) plot the txy graph and show states a, b and c. the graph must be done by computer and should be properly annotated. ethyl propyl ether (1) - chloroform (2) at 0.5 bar t ( ä±c) x1 y1 t ( ä±c) x1 y1 42.9 0.000 0.000 49.0 0.470 0.455 43.0 0.020 0.010 49.1 0.520 0.520 43.9 0.065 0.029 48.9 0.567 0.592 45.4 0.156 0.089 48.3 0.652 0.720 46.4 0.215 0.142 47.6 0.745 0.815 47.6 0.296 0.223 46.7 0.822 0.872 48.3 0.362 0.302 45.7 0.907 0.937 48.7 0.410 0.375 44.6 1.000
Answers: 3

Chemistry, 22.06.2019 05:30, madisonrosamond99
Astudent carefully transfers 30 g of water and 30 g of alcohol in a glass tube, forming two layers and filling the tube completely. after sealing the tube, the student mixes the solutions, and notices a bubble that forms in the tube. what is the mass of the contents in the glass tube after mixing?
Answers: 2

Chemistry, 22.06.2019 14:30, villarrealc1987
In water, a strong acid will break down into its component parts. a. completely b. partly c. never in water, a weak base will break down into its component parts. a. completely b. partly c. never
Answers: 2
Do you know the correct answer?
What is the best name for the molecule below? (2 points)
a compound with a total of nin...
a compound with a total of nin...
Questions in other subjects:


Mathematics, 04.08.2019 07:30

Biology, 04.08.2019 07:30



Biology, 04.08.2019 07:30

English, 04.08.2019 07:30

History, 04.08.2019 07:30

Biology, 04.08.2019 07:30

Biology, 04.08.2019 07:30