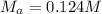Chemistry, 18.01.2020 09:31, Sumthin4695
A29.00 ml sample of an unknown h3po4 solution is titrated with a 0.130 m naoh solution. the equivalence point is reached when 27.73 ml of naoh solution is added.

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 10:00, aschool2000
Water's surface tension and heat storage capacity are accounted for by its a) orbitals b) weight c) hydrogen bonds d) mass e) size
Answers: 2


Chemistry, 22.06.2019 11:50, hamidaakter936848
Calculate the molarity of each of the following solutions. part a) 0.12 mol of lino3 in 5.5 l of solution part b) 60.7 g c2h6o in 2.48 l of solution part c) 14.2 mg ki in 100 ml of solution
Answers: 2
Do you know the correct answer?
A29.00 ml sample of an unknown h3po4 solution is titrated with a 0.130 m naoh solution. the equivale...
Questions in other subjects:

Social Studies, 26.06.2020 15:01

Mathematics, 26.06.2020 15:01

Mathematics, 26.06.2020 15:01

Spanish, 26.06.2020 15:01

Mathematics, 26.06.2020 15:01


Spanish, 26.06.2020 15:01




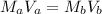 which is the titration formula. M is molarity (a is of acid and b is of base) and V is volume in mL (a is of acid and b is of base). Plugging in gives us
which is the titration formula. M is molarity (a is of acid and b is of base) and V is volume in mL (a is of acid and b is of base). Plugging in gives us 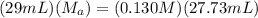 . Solving gives us
. Solving gives us