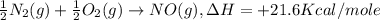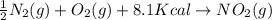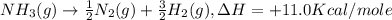Chemistry, 09.01.2020 06:31, MysteryDove12
Select all of the following reactions that are endothermic.
-h2(g) + ½o2(g) → h2o(g), δh = -57.83 kcal/mole
-½n2(g) + ½o2(g) → no(g), δh = +21.6 kcal/mole
-½n2(g) + o2(g) + 8.1 kcal → no2(g)
-½n2(g) + 3/2h2(g) → nh3(g) + 11.0 kcal/mole
-nh3(g) → ½n2(g) + 3/2h2(g), δh = +11.0 kcal/mole

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:00, ayoismeisalex
Matches the chemical name of each oxide of phosphorus to its chemical formula
Answers: 2

Chemistry, 22.06.2019 05:50, Makoshark6887
According to coulomb's law, how would the electrical force between particles change if the product of their electrical charge increased?
Answers: 1

Chemistry, 22.06.2019 10:30, officialalex6330
Balance and in which category does it fit in? single or double displacement or synthesis or decomposition? (a) k2 o → k + o2 (b) na + i2 → nai (c) cu(no3 )2 + naoh → cu(oh)2 + nano3 (d) kclo3 → kcl + o2 (e) ca(no3 )2 + hbr → cabr2 + hno3 (f) sn(oh)2 → sno + h2 o (g) p4 + n2 o → p4 o6 + n2 (h) fe + al2 (so4 )3 → feso4 + al (i) alcl3 + na2 co3 → al2 (co3 )3 + nacl (j) c3 h6 + o2 → co2 + h2 o
Answers: 1

Chemistry, 22.06.2019 12:30, ethanw8973
If 22.5 liters of oxygen reacted with excess of hydrogen, how many liters of water vapor could be produced?
Answers: 3
Do you know the correct answer?
Select all of the following reactions that are endothermic.
-h2(g) + ½o2(g) → h2o(g), δh = -57...
-h2(g) + ½o2(g) → h2o(g), δh = -57...
Questions in other subjects:


History, 10.07.2019 00:30

English, 10.07.2019 00:30


Mathematics, 10.07.2019 00:30

Physics, 10.07.2019 00:30


History, 10.07.2019 00:30

Mathematics, 10.07.2019 00:30