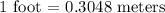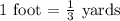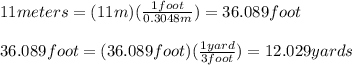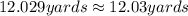Chemistry, 04.12.2019 23:31, zuberskylar
Zhang is designing protective packaging. he tests his design by dropping a raw egg protected by his packaging from various heights. he finds that the egg is protected up to a height of 11 meters. zhang needs to convert the height from meters to yards for his report. unit of length customary system units metric system units 1 inch 2.54 centimeters 1 foot 0.3048 meters 1 mile 1.61 kilometers how many yards can the packaged egg drop without breaking? use the table to find the answer. round to the nearest hundredth. 10.06 yards 12.03 yards 18.04 yards 36.09 yards

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 23.06.2019 07:30, shealynh52
Achemist at a pharmaceutical company is measuring equilibrium constants for reactions in which drug candidate molecules bind to a protein involved in cancer. the drug molecules bind the protein in a 1: 1 ratio to form a drug-protein complex. the protein concentration in aqueous solution at 25 ˚c is 1.74 x10-6 m . drug a is introduced into the protein solution at an initial concentration of 2.00 x10-6m. drug b is introduced into a separate, identical protein solution at an initial concentration of 2.00 x10-6m. at equilibrium, the drug a-protein solution has an a-protein complex concentration of 1.00 x10-6m, and the drug b solution has a b-protein complex concentration of 1.40 x10-6m. a. calculate the kc value for the a-protein binding reaction. b. calculate the kc value for the b-protein binding reaction. c. assuming that the drug that binds more strongly will be more effective, which drug is the better choice for further research?
Answers: 1

Chemistry, 23.06.2019 14:00, GreenHerbz206
During an acid-base titration, when do the contents of the beaker consist of only water, a salt, and a trace of indicator?
Answers: 2

Chemistry, 23.06.2019 14:00, kealinwiley
Which of the following is not a result when a change to an equilibrium system is applied? (2 points) increasing the rate of the forward reaction will cause a shift to the left. increasing the rate of the reverse reaction will cause a shift to the left. decreasing the rate of the forward reaction will cause a shift to the left. decreasing the rate of the reverse reaction will cause a shift to the right.
Answers: 1
Do you know the correct answer?
Zhang is designing protective packaging. he tests his design by dropping a raw egg protected by his...
Questions in other subjects:

Mathematics, 29.08.2019 07:50

History, 29.08.2019 07:50

English, 29.08.2019 07:50


English, 29.08.2019 07:50


Mathematics, 29.08.2019 07:50