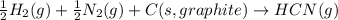Chemistry, 29.01.2020 00:51, marialandingin7520
Write a balanced chemical equation for the standard formation reaction of gaseous hydrogen cyanide hcn .

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:30, lasagnafoe
Used the balanced equation 2h2+ o2 - -> 2h2o. if you have 7.2 grams of o2 , how many grams of h2o can you produce ?
Answers: 2

Chemistry, 22.06.2019 04:30, anthony4034
Use the periodic table to determine the electron configuration of dysprosium (dy) and americium (am) in noble-gas notation.
Answers: 1

Chemistry, 22.06.2019 06:30, yolo123321
The following reaction shows sodium carbonate reacting with calcium hydroxide. na2co3 + ca(oh)2 → naoh + caco3 how many grams of naoh are produced from 20.0 grams of na2co3? (molar mass of na = 22.989 g/mol, c = 12.01 g/mol, o = 15.999 g/mol, ca = 40.078 g/mol, h = 1.008 g/mol) 12.2 grams 15.1 grams 24.4 grams 30.2 grams
Answers: 2

Chemistry, 23.06.2019 00:00, kittenalexis68
How many atoms or molecules are there in a mole of a substance?
Answers: 1
Do you know the correct answer?
Write a balanced chemical equation for the standard formation reaction of gaseous hydrogen cyanide h...
Questions in other subjects:


Physics, 09.12.2020 17:20

Chemistry, 09.12.2020 17:20

Mathematics, 09.12.2020 17:20

Physics, 09.12.2020 17:20




Arts, 09.12.2020 17:20