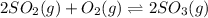Consider the reaction.
2so2(g)+o2(g) < > 2so3(g)
when does the given...

Consider the reaction.
2so2(g)+o2(g) < > 2so3(g)
when does the given chemical system reach dynamic equilibrium?
when the forward and reverse reactions stop
when the rate of the forward reaction is higher than the rate of the reverse reaction
when the concentration of the reactants is higher than the concentration of the products
when the rates of the forward and reverse reactions are equal

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:50, JuniperGalaxy
Consider the equilibrium system: 2icl(s) ⇄ i2(s) + cl2(g) which of the following changes will increase the total amount of of cl2 that can be produced? all of the listed answers are correct decreasing the volume of the container removing the cl2 as it is formed adding more icl(s) removing some of the i2(s)
Answers: 1

Chemistry, 22.06.2019 06:30, backup5485
Three cards with holes are arranged in a straight line. a light is shined through the first card’s hole and travels through all three cards. what does this tell you about light rays? a) that light is reflected b) that light is refractive c) that light travels in a straight line d) that light does not travel in a straight line
Answers: 1


Chemistry, 22.06.2019 14:30, Kiaraboyd9366
Select all of the statements which are true. electrons are located in shells or orbits around the atom. electrons orbit slowly around the atom. electrons travel in one flat path around the nucleus of an atom. the valence of an atom is determined by the number of electrons in the atom's outermost shell.
Answers: 1
Do you know the correct answer?
Questions in other subjects:

Mathematics, 28.04.2021 14:00

English, 28.04.2021 14:00

Mathematics, 28.04.2021 14:00


Physics, 28.04.2021 14:00



Mathematics, 28.04.2021 14:00


Mathematics, 28.04.2021 14:00