
Fluorine (f) and bromine (br) are in the same group on the periodic table. how do atoms of these elements compare when they form bonds?
1-both a fluorine atom and a bromine atom lose one electron, and both atoms become stable.
2-a fluorine atom becomes stable by losing one electron, but a bromine atom cannot become stable by losing only one electron.
3-both a fluorine atom and a bromine atom gain one electron, and both atoms become stable.
4-a fluorine atom becomes stable by gaining one electron, but a bromine atom cannot become stable by gaining only one electron.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:30, aydenmasonc
Which statement describes james chadwick’s discovery.
Answers: 2

Chemistry, 22.06.2019 18:00, kamjay2006
The human activities in two locations are described below: location a: rampant use of plastic containers location b: excessive use of pesticides and fertilizers which statement is most likely true? location a will have poor air quality because plastic is biodegradable. location a will experience water scarcity because plastic absorbs moisture. the population of honeybees will increase in location b because production of crops will increase. the population of fish in location b will decrease because the water is contaminated.
Answers: 1

Chemistry, 22.06.2019 22:30, lanashanabJHsbd1099
Who discovered a pattern to the elements in 1869?
Answers: 1
Do you know the correct answer?
Fluorine (f) and bromine (br) are in the same group on the periodic table. how do atoms of these ele...
Questions in other subjects:

Chemistry, 02.09.2019 06:30

Mathematics, 02.09.2019 06:30

Mathematics, 02.09.2019 06:30

World Languages, 02.09.2019 06:30

Social Studies, 02.09.2019 06:30



Mathematics, 02.09.2019 06:30



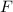 and bromine,
and bromine, 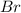 are in the same group on the periodic table that is halogen group (Group 17). The halogen group has electronic configuration as
are in the same group on the periodic table that is halogen group (Group 17). The halogen group has electronic configuration as 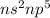 that means they are deficient of one electron to complete their octet and attain stability. So, they gain 1 electron and result in the formation of anion and form compounds by transfer of electrons.
that means they are deficient of one electron to complete their octet and attain stability. So, they gain 1 electron and result in the formation of anion and form compounds by transfer of electrons. 




