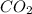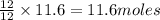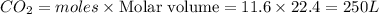Chemistry, 27.08.2019 18:00, cassiuspricerules
In the following reaction, how many liters of carbon dioxide will be produced if
250 liters of oxygen is used in the combustion of sucrose, given that both gases are at stp?
c12h22o11 + 12o2 → 12co2 + 11h2o
a. 125 liters b. 500 liters c. 250 liters d. 268.8 liters

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 12:30, meghan2529
The melting point of sulfur is 115 °c and its boiling point is 445 °c. what state would sulfur be in at 200 °c?
Answers: 1

Chemistry, 22.06.2019 16:40, roderickhinton
The diagram below shows the movement of particles. what does this piece of evidence best support? the collision theory the maxwell-boltzmann distribution the effect of pressure on reaction rates the effect of temperature on reaction rates
Answers: 3

Chemistry, 22.06.2019 17:00, davisnaziyahovz5sk
The arrangement of particles is most ordered in a sample of
Answers: 1
Do you know the correct answer?
In the following reaction, how many liters of carbon dioxide will be produced if
250 liters of...
250 liters of...
Questions in other subjects:




History, 24.06.2020 04:01






Mathematics, 24.06.2020 04:01


 of particles.
of particles.
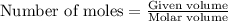
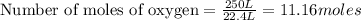
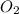 gives= 12 moles of
gives= 12 moles of