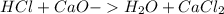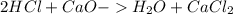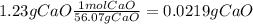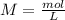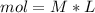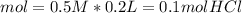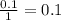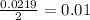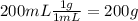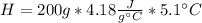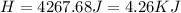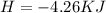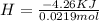During an experiment, a student adds 1.23 g of cao to 200.0 ml of 0.500 m hcl. the student observes a temperature increase of 5.10 °c. assuming the solution\'s final volume is 200.0 ml, the density if 1.00 g/ml, and the heat capacity is 4.184 j/(g·°c, calculate the heat of the reaction, ? hrxn.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:10, fvmousdiana
Determine the percent sulfuric acid by mass of a 1.61 m aqueous solution of h2so4. %
Answers: 2


Chemistry, 22.06.2019 04:30, ajsoccer1705
Using the periodic table, complete the table to describe each atom. type in your answers
Answers: 3
Do you know the correct answer?
During an experiment, a student adds 1.23 g of cao to 200.0 ml of 0.500 m hcl. the student observes...
Questions in other subjects:

Mathematics, 23.02.2021 21:00

Mathematics, 23.02.2021 21:00

Mathematics, 23.02.2021 21:00



History, 23.02.2021 21:00

Chemistry, 23.02.2021 21:00


Law, 23.02.2021 21:00

Mathematics, 23.02.2021 21:00


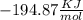
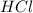 and
and 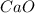 will produce
will produce 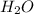 and
and 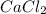 plus energy, because is an exothermic reaction. The first step is step up the reaction:
plus energy, because is an exothermic reaction. The first step is step up the reaction: