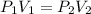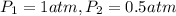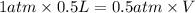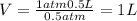Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 06:30, vlactawhalm29
Select the correct text in the passage. which sentences describe examples of sustainable living? i live in an old apartment building downtown, but my company is based in an office park on the outskirts of the city. i drive an old car that needs to be replaced. i plan to buy a hybrid for better gas mileage, but for now i am able to carpool with a couple of friends from work. the drive to the office park is about 45 minutes each way, but we do get to work in a modern building. the architects just received a leed certification for the design.
Answers: 3

Chemistry, 22.06.2019 14:00, jivsf
The two naturally occurring isotopes of chlorine are 35cl (34.969 amu, 75.77%) and 37cl (36.966 amu, 24.23%). the two naturally occurring isotopes of bromine are 79br (78.918 rm amu, 50.69%) and 81br (80.916 amu, 49.31%). chlorine and bromine combine to form bromine monochloride, brcl. 1. how many peaks will be present in a mass spectrum for brcl? the four combinations of molecule possible given these four isotopes are: 81br37cl, 81br35cl, 79br37cl, and 79br35cl. 2. what are the masses of the four different brcl molecules? express the masses using six significant figures, in decreasing numeric order (highest to lowest), separated by commas.
Answers: 3

Chemistry, 22.06.2019 17:00, princessakosua2
The msds for glacial acetic acid says that it is a flammable liquid that can severely burn any human tissue it comes in contact with. it reacts with bases, various metals, and strong oxidizing agents. its vapors can form explosive mixtures with air.
Answers: 1
Do you know the correct answer?
Aballoon filled with helium gas has a volume of 500 ml at a pressure of 1 atm. the balloon is releas...
Questions in other subjects:



Mathematics, 03.04.2020 04:08

Mathematics, 03.04.2020 04:08

Mathematics, 03.04.2020 04:08


Health, 03.04.2020 04:08


Mathematics, 03.04.2020 04:08


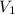 (1000 mL= 1 L)
(1000 mL= 1 L)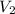
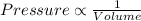 (At constant temperature)
(At constant temperature)