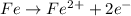In a chemical reaction, an iron atom became the ion fe2+. what happened to the iron atom?
a....

Chemistry, 23.01.2020 16:31, Erinkim2292
In a chemical reaction, an iron atom became the ion fe2+. what happened to the iron atom?
a. it lost electrons and was oxidized.
b. it lost electrons and was reduced.
c. it gained electrons and was oxidized.
d. it gained electrons and was reduced.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 18:10, NEONREDBLADE
Areader can tell that the meaning of “obnoxious” will include “having the quality of something” because of the .a) prefix b)pronunciation c)suffix d) word root
Answers: 3

Chemistry, 23.06.2019 15:00, ericperkins10ox0b27
In two or more complete sentences describe all of the van der waals forces that exist between molecules of sulfur dioxide, so2.
Answers: 1

Chemistry, 23.06.2019 16:00, lucy773
Henry moseley used x-ray experiments to determine the atomic number of elements. how did his discovery contribute to the development of the periodic table? a. it confirmed that elements should be arranged in strict order of increasing atomic mass. b. it led to elements with similar atomic numbers being grouped together. c. it allowed the elements to be placed in strict order of increasing atomic number. d. it showed that the way mendeleev grouped elements together was completely wrong.
Answers: 1

Chemistry, 23.06.2019 23:30, emwvoidsnake
When the reaction 2h2s(g) 2h2(g) + s2(g) is carried out at 1065°c, kp = 0.012. starting with pure h2s at 1065°, what must the initial pressure of h2s be if the equilibrated mixture at this temperature is to contain 0.250 atm of h2(g)?
Answers: 1
Do you know the correct answer?
Questions in other subjects:







English, 25.02.2020 22:29




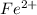 which means that iron atom by loosing two electrons from its valence shell has oxidized into positively charged ion.
which means that iron atom by loosing two electrons from its valence shell has oxidized into positively charged ion.