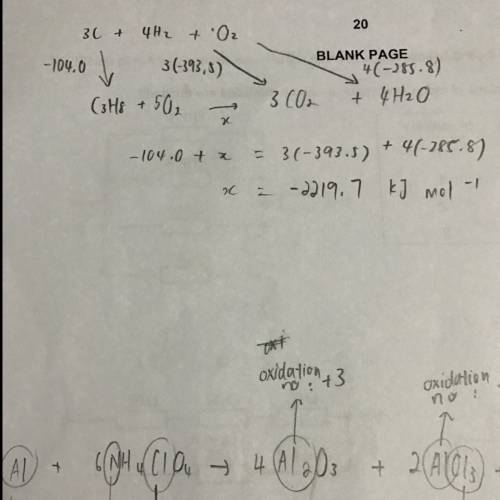
c(s) + o2(g) → co2(g) ∆h = −393.5 kj mol−1

Chemistry, 02.09.2019 16:00, emily200705
Use the information below to answer this question
c(s) + o2(g) → co2(g) ∆h = −393.5 kj mol−1
h2(g) + o2(g) → h2o(l) ∆h = −285.8 kj mol−1
3c(s) + 4h2(g) → c3h8(g) ∆h = −104.0 kj mol−1
4c(s) + 5h2(g) → c4h10(g) ∆h = −125.2 kj mol−1
the value in kj mol−1
for the enthalpy of combustion of propane is

Answers: 1
Similar questions

Social Studies, 02.07.2019 02:30, hcameron65
Answers: 1

Business, 15.07.2019 12:00, genyjoannerubiera
Answers: 1

History, 23.07.2019 13:00, alexciamartinez05
Answers: 1

Computers and Technology, 30.09.2019 08:20, ajsoccer1705
Answers: 1
Do you know the correct answer?
Use the information below to answer this question
c(s) + o2(g) → co2(g) ∆h = −393.5 kj mol−1
c(s) + o2(g) → co2(g) ∆h = −393.5 kj mol−1
Questions in other subjects:






Mathematics, 18.06.2020 00:57

Health, 18.06.2020 00:57

English, 18.06.2020 00:57