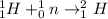Chemistry, 11.01.2020 04:31, npaslayoy1bzj
Examine the nuclear reaction: (1/1)h+(1/0)n--> (2/1)h. why is this classified as a nuclear reaction rather than a chemical reaction?
it is not balanced.
a new compound is formed.
a change has occurred in a nucleus.
a new element has been formed.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:00, mariamakonteh31
Will give ! what are the advantages and disadvantages of nuclear power? check all that apply. one advantage of nuclear energy is that it does not produce carbon dioxide emissions. storage of nuclear waste is a short-term problem associated with nuclear energy. the problem with uranium mining is that a large quantity of uranium must be extracted to meet energy needs because the energy release from uranium fission is so low. safe operation of a nuclear power plant can be jeopardized by a human mistake.
Answers: 1


Chemistry, 22.06.2019 15:10, strodersage
Which statement describes the phase change that occurs when dry ice is placed in an open container at room temperature?
Answers: 1

Chemistry, 22.06.2019 15:30, 20cschultz
Which suspect most likely committed the robbery and how do you know
Answers: 2
Do you know the correct answer?
Examine the nuclear reaction: (1/1)h+(1/0)n--> (2/1)h. why is this classified as a nuclear react...
Questions in other subjects:

Mathematics, 18.09.2019 01:00

Mathematics, 18.09.2019 01:00

Mathematics, 18.09.2019 01:00

Social Studies, 18.09.2019 01:00





Mathematics, 18.09.2019 01:00