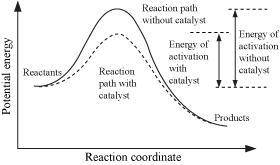
Chemistry, 19.09.2019 14:50, makennskyee1198
The action of a catalyst can be explained in the following manner: the catalyst lowers the temperature of the reactants. the catalyst takes no part in the reaction but serves as a buffer between reactants and products. the catalyst prevents the reverse reaction. the catalyst makes it possible for the reaction to take place by another path that makes possible reaction at a lower energy.

Answers: 2
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 12:00, Unknowndragon42
Consider the following reaction at equilibrium. 2co2 (g) 2co (g) + o2 (g) h° = -514 kj le châtelier's principle predicts that the equilibrium partial pressure of co (g) can be maximized by carrying out the reaction a. at high temperature and high pressure b. at high temperature and low pressure c. at low temperature and low pressure d. at low temperature and high pressure e. in the presence of solid carbon
Answers: 2

Chemistry, 23.06.2019 00:30, mathwiznot45
Element j is 1s 2s 2p 3s . (i) how many unpaired electrons does j have? (ii) is j a good oxidizing agent or a reducing agent? (iii) state reason for the answer.
Answers: 1
Do you know the correct answer?
The action of a catalyst can be explained in the following manner: the catalyst lowers the temperat...
Questions in other subjects:

Mathematics, 04.11.2021 19:40

Biology, 04.11.2021 19:40

Mathematics, 04.11.2021 19:40



Mathematics, 04.11.2021 19:40


English, 04.11.2021 19:40