The images show two processes. image a: a roasting marshmallow, image b: melting ice. which statement about the two images is true?
1. image a shows a physical change, and image b shows a chemical reaction.
2. image a shows a chemical reaction, and image b shows a physical change.
3. both images show chemical reactions that are endothermic.
4. both images show chemical reactions that are exothermic.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 18:00, tatemelliott
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 2

Chemistry, 22.06.2019 21:30, starl0rd211
Describe at least two advantages and two disadvantages of using hydropower as a source of energy.
Answers: 2


Chemistry, 23.06.2019 13:00, Crxymia
How long could you survive without electricity? what parts of your life would be affected by loss of electricity? should you prepare for an electricity outage, and if so, how would you prepare? what backup system could your family or community install to generate limited amounts of electricity during an outage? how does this system create an electric force field and generate electric current?
Answers: 2
Do you know the correct answer?
The images show two processes. image a: a roasting marshmallow, image b: melting ice. which statem...
Questions in other subjects:

Geography, 10.05.2021 16:50


Mathematics, 10.05.2021 16:50



English, 10.05.2021 16:50


Social Studies, 10.05.2021 16:50


Mathematics, 10.05.2021 16:50


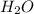 from solid to liquid form and thus is a physical change.
from solid to liquid form and thus is a physical change.