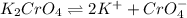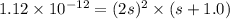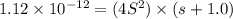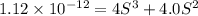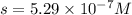Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 04:00, amandasantiago2001
Which of the following ocean acidification? are the most likely side effects of a ph less than 7.0 in the ocean b. more metal salts altering the ocean chemistry c. dissolution of the shells of marine organisms d. both a & b e. all of the above.
Answers: 3

Chemistry, 22.06.2019 04:00, heavyhearttim
4. absorption has the highest risk of overdose due to increased potency. a. rectal b. oral c. transdermal d. intranasal
Answers: 2

Chemistry, 22.06.2019 05:00, adjjones2011
As you watch a surfer ride a wave towards the shoreline, what is the shoreline? a) displacement reference b) reference point c) coordinate plane d) cartesian boundary
Answers: 1
Do you know the correct answer?
Silver chromate is sparingly soluble in aqueous solutions. the ksp of ag2cro4 is 1.12× 10–12. what i...
Questions in other subjects:



Mathematics, 13.04.2020 03:38

Mathematics, 13.04.2020 03:38

English, 13.04.2020 03:38


Mathematics, 13.04.2020 03:38

Mathematics, 13.04.2020 03:38

History, 13.04.2020 03:39


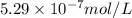
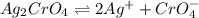
![K_{sp}=[Ag^{+}]^2[CrO_4^{-}]](/tpl/images/0248/2494/607c1.png)
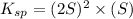
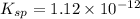
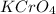 is written as:
is written as: