
Chemistry, 05.10.2019 18:30, sportygirlscand
Calculate the percent ionization of nitrous acid in a solution that is 0.139 m in nitrous acid. the acid dissociation constant of nitrous acid is 4.50 ⋅ 10-4.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:50, deanlmartin
Choose all that apply. when creating a graph, you should: determine the x- and y- variables label the scale on the x- and y- axes plot the data points draw a line of best fit to represent the data trend
Answers: 1

Chemistry, 22.06.2019 00:30, thatonestudent2271
If 3.00 g of titanium metal is reacted with 6.00 g of chlorine gas, cl2, to form 7.7 g of titanium (iv) chloride in a combination reaction, what is the percent yield of the product?
Answers: 1

Chemistry, 22.06.2019 05:50, zaleemawhite
Significant figures are digits read directly from the measuring instrument plus one more digit, which is __ by the observer.
Answers: 2
Do you know the correct answer?
Calculate the percent ionization of nitrous acid in a solution that is 0.139 m in nitrous acid. the...
Questions in other subjects:


English, 25.03.2020 01:47

Mathematics, 25.03.2020 01:48

English, 25.03.2020 01:48


Biology, 25.03.2020 01:48


History, 25.03.2020 01:48



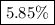 .
.
![{\text{K}}=\frac{{\left[{\text{D}}\right]\left[{\text{C}}\right]}}{{\left[{\text{A}}\right]\left[ {\text{B}}\right]}}](/tpl/images/0290/4975/92f42.png)
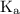 and equilibrium constant for the dissociation of base is known as
and equilibrium constant for the dissociation of base is known as 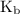 .
.![\% {\text{ ionization}}=\frac{{\left[ {{{\text{H}}_{\text{3}}}{{\text{O}}^{\text{ + }}}}\right]}}{{\left[{{\text{HA}}}\right]}}\times100](/tpl/images/0290/4975/5479a.png)
![\left[{{{\text{H}}_{\text{3}}}{{\text{O}}^{\text{+}}}}\right]](/tpl/images/0290/4975/53aa9.png) is the concentration of hydronium ion and
is the concentration of hydronium ion and ![\left[{{\text{HA}}}\right]](/tpl/images/0290/4975/88419.png) is the concentration of acid.
is the concentration of acid.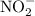 and hydronium ion.
and hydronium ion.
![\% {\text{ ionization}}=\frac{{\left[{{{\text{H}}_{\text{3}}}{{\text{O}}^{\text{+}}}}\right]}}{{\left[{{\text{HN}}{{\text{O}}_2}}\right]}}\times100](/tpl/images/0290/4975/29d81.png) …… (2)
…… (2) .
.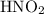 becomes 0139-x at equilibrium. The concentration of
becomes 0139-x at equilibrium. The concentration of 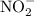 and
and 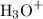 ion becomes x at equilibrium.
ion becomes x at equilibrium.![\left[{{\text{NO}}_2^-}\right]](/tpl/images/0290/4975/bb69c.png) and 0.139-x for
and 0.139-x for 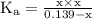 …… (3)
…… (3)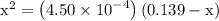
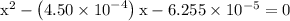
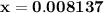
![\left[{{\text{HN}}{{\text{O}}_2}}\right]](/tpl/images/0290/4975/958ed.png) in equation (2).
in equation (2).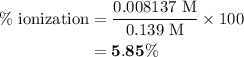
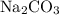 (aq):
(aq): 



