Chemistry, 17.12.2019 09:31, dextor1606
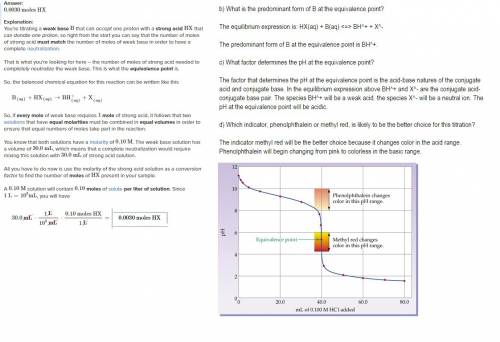
Assume that 30.0 ml of a 0.10 m solution of a weak base b that accepts one proton is titrated with a 0.10 m solution of a monoprotic strong acid hx. (a) how many moles of hx have been added at the equivalence point? (b) what is the predominant form of b at the equivalence point? (c) what factor determines the ph at the equivalence point? (d) which indicator, phenolphthalein or methyl red, is likely to be the better choice for this titration?

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:50, mrylenastewart
What are transitions between a liquid and a solid called? identify which way they are transitioning
Answers: 2

Chemistry, 22.06.2019 06:00, rebeccacruzz2017
Calculate - analysis of compound composed of iron and oxygen yields 174.86 of fe and 75.14g of o. what is the empirical formula for this compound?
Answers: 3

Chemistry, 22.06.2019 09:00, kcarstensen59070
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1
Do you know the correct answer?
Assume that 30.0 ml of a 0.10 m solution of a weak base b that accepts one proton is titrated with a...
Questions in other subjects:



History, 22.01.2021 22:40

Mathematics, 22.01.2021 22:40

Mathematics, 22.01.2021 22:40


Mathematics, 22.01.2021 22:40



Mathematics, 22.01.2021 22:40