Chemistry, 13.12.2019 17:31, jladinosolarsee
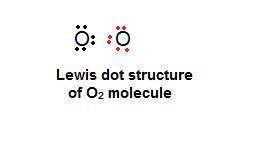
What best explains how two oxygen atoms, each with six valence electrons, can bond with each other?
one atom can lose two electrons so that the other atom can gain them and have eight valence electrons.
one atom can lose four electrons to the environment so that a total of eight valence electrons remains.
each atom can share two electrons with the other so that each atom has eight valence electrons.
each atom can lose two electrons so that there is a total of eight valence electrons between the atoms.

Answers: 2
Other questions on the subject: Chemistry




Chemistry, 23.06.2019 01:30, koggebless
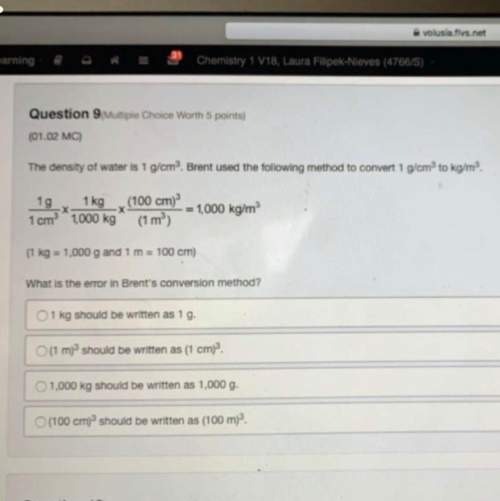
The solubility of barium nitrate is 9.02 g/100 g h2o at 20°c. a 15.2 g sample of barium nitrate is added to 200.0 g of water at 20°c. is the solution saturated, unsaturated, or supersaturated? a. unsaturated b. saturated c. supersaturated
Answers: 1
Do you know the correct answer?
What best explains how two oxygen atoms, each with six valence electrons, can bond with each other?...
Questions in other subjects:

English, 30.12.2019 02:31

History, 30.12.2019 02:31

Mathematics, 30.12.2019 02:31

History, 30.12.2019 02:31



Mathematics, 30.12.2019 03:31


Spanish, 30.12.2019 03:31

Mathematics, 30.12.2019 03:31


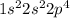
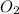 molecule is given in the image attached.
molecule is given in the image attached.