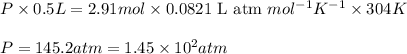Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:50, suzymott1562
Nitrogen dioxide reacts with water to form nitric acid and nitrogen monoxide according to the equation: 3no2(g)+h2o(l)→2hno3(l)+no(g) part a suppose that 4.2 mol no2 and 0.50 mol h2o combine and react completely. which reactant is in excess? express your answer as a chemical formula. nothing
Answers: 1

Chemistry, 22.06.2019 08:30, omoaye
Identify one disadvantage to each of the following models of electron configuration: -dot structures -arrow and line diagrams -written electron configurations type in your answer below. (answer) -dot structures do not show the distribution of electrons in orbitals and take up a lot of space. -arrow and line diagrams take up a lot of space and make it difficult to count electrons. -written configurations make it easy to lose count of electrons and do not show the distribution of electrons in orbitals.
Answers: 3

Chemistry, 23.06.2019 03:30, alvfran1041
Astudent uses universal ph paper to find the ph of three solutions . solution a has a ph of 5 solution b has a ph of 11 and solution c has a ph of 7 identify which solution is acidic which solution is neutral and which solution is basic
Answers: 1

Chemistry, 23.06.2019 03:30, damyonfenton13
If 2 molecules of one reactant combine with 3 molecules of another to produce 5 molecules of a product, then what is the representation of the reaction?
Answers: 1
Do you know the correct answer?
Given 2.91 moles of a gas in a 500 milliliter-container, if the temperature is found to be 31 degree...
Questions in other subjects:

Social Studies, 16.12.2020 22:30




Mathematics, 16.12.2020 22:30

History, 16.12.2020 22:30

Arts, 16.12.2020 22:30

English, 16.12.2020 22:30

Mathematics, 16.12.2020 22:30


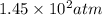
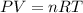
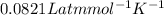
![31^oC=[273+31]K=304K](/tpl/images/0304/4872/a160b.png)