The transition from n = 3 to n = 5 energy level is an  .
.
The transition from n = 1 to n = 3 energy level is an .
.
The transition from n = 3 to n = 2 energy level is an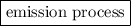 .
.
The transition from n = 2 to n = 1 energy level is an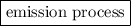 .
.
Further explanation:
An electronic transition is a process that occurs when an electron undergoes emission or absorption from one energy level to another energy level.
When an electron undergoes a transition from a lower energy level to a higher energy level then it requires energy to complete the process. This transition is an absorption process.
When an electron undergoes a transition from higher energy level to lower energy level then it emits energy to complete the process. This transition is an emission process.
The hydrogen atoms are dissociated when an electric discharge is passed through its molecules. As a result, electromagnetic radiations are emitted by the excitation of hydrogen atoms. The hydrogen spectrum contains radiations of different frequencies.
The formula to calculate the energy of transition in the hydrogen atom is as follows:
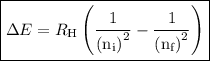
Here,
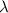 is the wavelength of transition.
is the wavelength of transition.
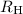 is Rydberg constant.
is Rydberg constant.
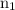 is the initial energy level of transition.
is the initial energy level of transition.
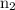 is the final energy level of transition.
is the final energy level of transition.
When the transition occurs from the first energy level to any other level, it is termed as Lyman series.
When the transition occurs from the second energy level to any other level, it is termed as Balmer series.
When the transition occurs from the third energy level to any other level, it is termed as Paschen series.
When the transition occurs from the fourth energy level to any other level, it is termed as Brackett series.
When the transition occurs from the fifth energy level to any other level, it is termed as Pfund series.
n = 3 to n = 5
Here, the electron goes from the lower energy level (n = 3) to a higher energy level (n = 5), so energy is absorbed in this process. Hence, this transition is classified as an absorption process.
n =1 to n = 3
Here, the electron goes from the lower energy level (n = 1) to a higher energy level (n = 3), so energy is absorbed in this process. Hence, this transition is classified as an absorption process.
n = 3 to n = 2
Here, the electron goes from higher energy level (n = 3) to lower energy level (n = 2), therefore energy is emitted in this process. Hence, this transition is classified as an emission process.
n = 2 to n = 1
Here, the electron goes from higher energy level (n = 2) to lower energy level (n = 1), therefore energy is emitted in this process. Hence, this transition is classified as an emission process.
Learn more:
1. Which transition is associated with the greatest energy change?
2. Describe the spectrum of elemental hydrogen gas:
Answer details:
Grade: Senior School
Subject: Chemistry
Chapter: Atomic structure
Keywords: hydrogen spectrum, Lyman, Balmer, Paschen, Brackett, Pfund, RH, ni, nf, first, second, third, fourth, fifth, emission, absorption.
















 .
.
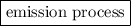 .
.
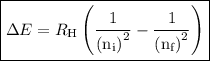
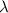 is the wavelength of transition.
is the wavelength of transition.
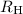 is Rydberg constant.
is Rydberg constant.
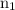 is the initial energy level of transition.
is the initial energy level of transition.
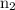 is the final energy level of transition.
is the final energy level of transition.




