
Chemistry, 28.01.2020 21:58, alaina3792
When a person has excess stomach acid, he or she may ingest some sodium bicarbonate. the rationale for doing this is that the sodium bicarbonate is:
a base and will neutralize the excess acid
an acid and will weaken the excess acid
neutral and will dilute the excess acid
it will make the person thirsty; they will drink water and dilute the excess acid.

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 21.06.2019 22:30, erinxmeow8
What are the charges of the subatomic particles by choosing the answer from the drop down menu. protons have a (+1,0,or-1). (protons, neutrons, electrons) have a 0 charge. 3.) electrons have a (+1,0,-1)
Answers: 2

Chemistry, 22.06.2019 02:30, caeyanij
When svante arrhenius first proposed his acid-base theory, he was a doctoral candidate. his professors thought his ideas were unfounded. within a decade, the arrhenius theory of acid-base was widely accepted and praised within the scientific world. arrhenius defined acids as compounds having ionizable hydrogen and bases as compounds with ionizable a) barium. b) hydronium. c) hydroxide. d) oxygen.
Answers: 3
Do you know the correct answer?
When a person has excess stomach acid, he or she may ingest some sodium bicarbonate. the rationale f...
Questions in other subjects:

English, 26.10.2021 17:50



History, 26.10.2021 17:50

History, 26.10.2021 17:50



Chemistry, 26.10.2021 17:50


Mathematics, 26.10.2021 17:50


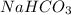 . It is basic in nature as it dissociates to give sodium and bicarbonate ions.
. It is basic in nature as it dissociates to give sodium and bicarbonate ions. 




