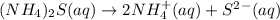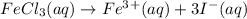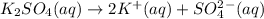Chemistry, 09.01.2020 03:31, amandasantiago2001
Which aqueous solution has the lowest boiling point?
a.0.20 m (nh4)2s
b. 0.20 m fei3
c. 0.20 m k2so4
d. 0.20 m c2h5oh

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:00, WhiteWinterRose
What is the chemical formula of the following compound
Answers: 3

Chemistry, 22.06.2019 05:20, jtingley0502
Temperature is _related to the average kinetic energy of a gas. inversely directly not disproportionally
Answers: 1

Chemistry, 22.06.2019 11:00, micro7909
Predict the products of the following acid-base reactions, and predict whether the equilibrium lies to the left or to the right of the reaction arrow. part ao2-(aq)+h2o(l)< => express your answer as part of a chemical equation. identify all of the phases in your answer. o2-(aq)+h2o(l) < => oh-(aq)+oh-(aq)part bpredict whether the equilibrium lies to the left or to the right of the equation in previous part. h2o is a stronger acid than oh–, so the equilibrium lies to the right. h2o is a weaker acid than oh–, so the equilibrium lies to the left. h2o is a stronger acid than oh–, so the equilibrium lies to the left. h2o is a weaker acid than oh–, so the equilibrium lies to the right. part cch3cooh(aq)+hs? (aq) < => express your answer as part of a chemical equation. identify all of the phases in your answer. ch3cooh(aq)+hs-(aq) < => h2s(aq)+c2h3o2-(aq)h2s(aq)+c2h3o2-( aq)part dpredict whether the equilibrium lies to the left or to the right of the equation in previous part. ch3cooh is a weaker acid than h2s, so the equilibrium lies to the right. ch3cooh is a weaker acid than h2s, so the equilibrium lies to the left. ch3cooh is a stronger acid than h2s, so the equilibrium lies to the right. ch3cooh is a stronger acid than h2s, so the equilibrium lies to the left. part eno2-(aq)+h2o(l) < => express your answer as part of a chemical equation. identify all of the phases in your answer. no2-(aq)+h2o(l) < => part fpredict whether the equilibrium lies to the left or to the right of the equation in previous part. hno2 is a stronger acid than h2o, so the equilibrium lies to the right. hno2 is a weaker acid than h2o, so the equilibrium lies to the left. hno2 is a stronger acid than h2o, so the equilibrium lies to the left. hno2 is a weaker acid than h2o, so the equilibrium lies to the right.
Answers: 1

Chemistry, 22.06.2019 14:00, rosetoheart2
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 2
Do you know the correct answer?
Which aqueous solution has the lowest boiling point?
a.0.20 m (nh4)2s
b. 0.20 m fei3...
a.0.20 m (nh4)2s
b. 0.20 m fei3...
Questions in other subjects:

Mathematics, 28.10.2020 17:10

Mathematics, 28.10.2020 17:10



Computers and Technology, 28.10.2020 17:10


SAT, 28.10.2020 17:10




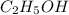

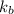 is the boiling point elevation constant.
is the boiling point elevation constant.