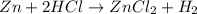When zinc is placed in hydrochloric acid, a gas is produced. when the gas is collected in a tube and a lit match is placed in the tube, a pop is heard. this is a test for hydrogen gas. what can be concluded from this observations. a) a change in state has occurred. b) the zinc metal was turned into hydrogen gas. c) a chemical change has occurred producing the hydrogen gas. d) a physical change has occurred producing the hydrogen gas.

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 07:30, reaperqueen21
Compare and contrast the bohr model and the electron cloud models of the atom.
Answers: 1

Chemistry, 22.06.2019 09:00, boxergirl2062
Scientific evidence tells us that the cause of earths four season is the tilt of earth as it revolves around the sun. the student is instructed to illustrate this information in a science notebook. how will the student illiterate winter in the northern hemisphere?
Answers: 3
Do you know the correct answer?
When zinc is placed in hydrochloric acid, a gas is produced. when the gas is collected in a tube and...
Questions in other subjects:


Biology, 13.10.2019 16:50

Mathematics, 13.10.2019 16:50

Biology, 13.10.2019 16:50



Social Studies, 13.10.2019 16:50

Chemistry, 13.10.2019 16:50

Mathematics, 13.10.2019 16:50