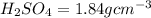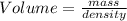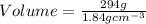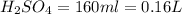2. sulfur dioxide gas (so2) reacts with excess oxygen gas (o2) and excess liquid water (h2o) to form liquid sulfuric acid (h2so4) in the unbalanced equation below: so2 + o2 + h2o h2so4 in the laboratory, a chemist carries out this reaction at stp with 67.2 l of sulfur dioxide (so2). how many liters of h2so4 did the chemist produce? 1 mole of any gas = 22.4 l of that same gas at stp • part a: write a balanced equation for the reaction. • part b: calculate the number of liters of h2so4 produced.

Answers: 2
Other questions on the subject: Chemistry




Chemistry, 23.06.2019 10:30, sbelgirl2000
If a 20.0ml test tube measures 15.0cm, what is the length in meters?
Answers: 1
Do you know the correct answer?
2. sulfur dioxide gas (so2) reacts with excess oxygen gas (o2) and excess liquid water (h2o) to form...
Questions in other subjects:



Mathematics, 05.10.2020 03:01





Mathematics, 05.10.2020 03:01

History, 05.10.2020 03:01


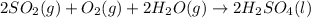
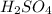 =0.16L
=0.16L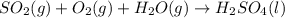
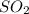 occupies 22.4 L at STP
occupies 22.4 L at STP 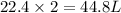 and produce 2 moles of
and produce 2 moles of 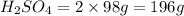
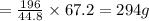 of
of