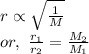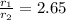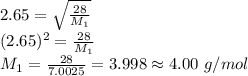Chemistry, 16.10.2019 17:40, cecilysimpson7521
Asample of nitrogen gas is contaminated with a gas (gas a) of unknown molar mass. the partial pressure of each gas is known to be 200 torr at 25°c. the gases are allowed to effuse through a pinhole, and it is found that gas a escapes 2.65 times the rate of nitrogen gas. what is the molar mass of gas a?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 17:30, kevin72937
Consider the story you just read. all but one of the choices below indicate that something is living.
Answers: 1

Chemistry, 23.06.2019 06:00, wirchakethan23
When hydrogen peroxide (h2o2) is added to potassium iodide (ki) solution, the hydrogen peroxide decomposes into water (h2o) and oxygen (o2). the chemical equation for the decomposition reaction is: 2h2o2—> 2h2o + o2. what is the role of the potassium iodide in this reaction? a. reactant. b. product. c. precipitate. d. catalyst.
Answers: 1

Chemistry, 23.06.2019 08:30, aambitiouss
Of element x has 22 protons, how many electrons does it have
Answers: 1

Chemistry, 23.06.2019 10:30, jetblackcap
An atom that gains or loses one or more electrons is called a(n)
Answers: 1
Do you know the correct answer?
Asample of nitrogen gas is contaminated with a gas (gas a) of unknown molar mass. the partial pressu...
Questions in other subjects:



Business, 16.07.2019 06:50







History, 16.07.2019 06:50