
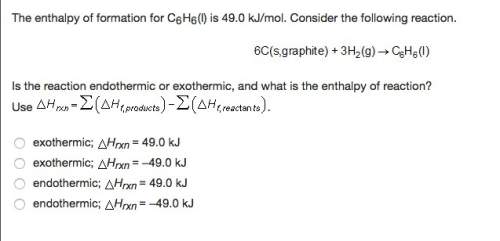
The enthalpy of formation for c6 h6 (i) is 49.0 kj/mol. consider the following reaction.
is the reaction endothermic or exothermic, and what is the enthalpy of reaction?
exothermic; mc014-3.jpghrxn = 49.0 kj
exothermic; mc014-4.jpghrxn = –49.0 kj
endothermic; mc014-5.jpghrxn = 49.0 kj
endothermic; mc014-6.jpghrxn = –49.0 kj

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, monnn91351
Determine the wavelength of the light absorbed when an electron in a hydrogen atom makes a transition from an orbital in the n=3 level to an orbital in the n=7 level.
Answers: 2

Chemistry, 23.06.2019 02:00, xoxoadara13ox07ck
Now look at the segment of the graph between the two data points marked with black squares. describe how the boiling point and melting point plots behave between these points. be as specific as possible.
Answers: 1

Do you know the correct answer?
The enthalpy of formation for c6 h6 (i) is 49.0 kj/mol. consider the following reaction.
...
...
Questions in other subjects:


Mathematics, 09.10.2019 00:30



History, 09.10.2019 00:30

History, 09.10.2019 00:30

Geography, 09.10.2019 00:30


English, 09.10.2019 00:30

Mathematics, 09.10.2019 00:30


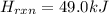
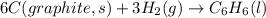
![\Delta H=\sum [n\times \Delta H_f(product)]-\sum [n\times \Delta H_f(reactant)]](/tpl/images/0345/6718/76c37.png)
![\Delta H=[(n_{C_6H_6}\times \Delta H_{C_6H_6})]-[(n_{H_2}\times \Delta H_{H_2})+(n_{C}\times \Delta H_{C})]](/tpl/images/0345/6718/5d909.png)
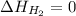 (as heat of formation of substances in their standard state is zero
(as heat of formation of substances in their standard state is zero
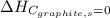 (as heat of formation of substances in their standard state is zero
(as heat of formation of substances in their standard state is zero
![\Delta H=[(1\times 49.0)]-[(3\times 0)+(6\times 0]](/tpl/images/0345/6718/8bb49.png)
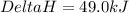
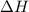 for the reaction comes out to be negative.
for the reaction comes out to be negative.





