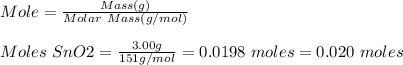Chemistry, 03.02.2020 21:02, alyssamaize
Base your answer to the question on the information below and on your knowledge of chemistry. at 1023 k and 1 atm, a 3.00-gram sample of sno2(s) (gram formula mass = 151 g/mol) reacts with hydrogen gas to produce tin and water, as shown in the balanced equation below. sno2(s) + 2h2(g) → sn(l) + 2h2o(g) show a numerical setup for calculating the number of moles of sno2(s) in the 3.00-gram sample.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 14:40, 4tazaouiamine1r
What type of solution is formed if 10 g of kclo3 are dissolved in 100g of water at 30
Answers: 2



Chemistry, 22.06.2019 15:00, levelebeasley1
Which theory was contradicted by experiments with the photoelectric effect? light spreads out after it passes through a small opening. as soon as light strikes metal, electrons will be ejected. visible light, regardless of color, will cause the ejection of electrons when striking metal. the kinetic energy of ejected electrons depends on the frequency of light that strikes the metal.
Answers: 2
Do you know the correct answer?
Base your answer to the question on the information below and on your knowledge of chemistry. at 102...
Questions in other subjects:




Mathematics, 19.03.2020 05:57



Mathematics, 19.03.2020 05:57


Social Studies, 19.03.2020 05:57

History, 19.03.2020 05:58