
Chemistry, 25.08.2019 07:30, sindy35111
Given a reaction mechanism that has an overall reaction of 2o3 mc010-1.jpg 3o2 and a rate of k[o3][o], which is the correct rate-determining step?
o3 + 2o mc010-2.jpg 3o2
o2 + o mc010-3.jpg o3
o3 mc010-4.jpg o2 + o
o3 + o mc010-5.jpg 2o2

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:00, daigle18383
Solar energy is energy from the sun that is converted into thermal or energy. a. nuclear b. mechanical c. electrical d. chemical
Answers: 2

Chemistry, 21.06.2019 22:00, braydentillery1221
If the particles in a sample of matter have an orderly arrangement and move only in place, the sample is a
Answers: 1

Chemistry, 22.06.2019 07:30, reaperqueen21
Compare and contrast the bohr model and the electron cloud models of the atom.
Answers: 1

Chemistry, 22.06.2019 08:30, ebigham5117
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 2
Do you know the correct answer?
Given a reaction mechanism that has an overall reaction of 2o3 mc010-1.jpg 3o2 and a rate of k[o3][o...
Questions in other subjects:

English, 12.12.2020 16:40








Social Studies, 12.12.2020 16:40


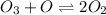
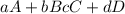
![Rate=k[A]^a[B]^b](/tpl/images/0195/8956/8c0b1.png)
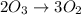
![k[O_3][O]](/tpl/images/0195/8956/9515b.png)
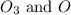 and the stoichiometry of the reactants is 1. Hence, the equation from which the rate is written is
and the stoichiometry of the reactants is 1. Hence, the equation from which the rate is written is 



