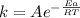Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:00, dustinsampsin2486
14. many depressants reduce small muscle control, making it harder for a. you to steer b. your mind to consider complex problems c. the eye to scan, focus, or stay still d. the kidneys to filter alcohol out of the bloodstream
Answers: 3

Chemistry, 22.06.2019 07:30, candigirl8847
All cells are made of four types of acids, lipids, proteins, and carbohydrates.
Answers: 1


Chemistry, 22.06.2019 19:30, Sumitco9578
Anurse used a 0.02-mg/l solution of disinfection to clean a patients wound. what is the concentration of the solution expressed as a percentage?
Answers: 1
Do you know the correct answer?
If a temperature increase from 12.0 ∘c to 22.0 ∘c doubles the rate constant for a reaction, what is...
Questions in other subjects:

Mathematics, 17.09.2020 09:01

Mathematics, 17.09.2020 09:01

Mathematics, 17.09.2020 09:01

Mathematics, 17.09.2020 09:01

Mathematics, 17.09.2020 09:01

Mathematics, 17.09.2020 09:01

Mathematics, 17.09.2020 09:01

Mathematics, 17.09.2020 09:01

Mathematics, 17.09.2020 09:01

Mathematics, 17.09.2020 09:01