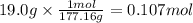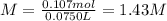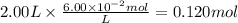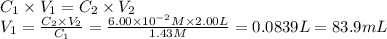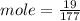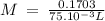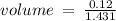On part "c": the forensic technician at a crime scene has just prepared a luminol stock solution by adding 19.0g of luminol into a total volume of 75.0ml of h2o.
a)what is the molarity of the stock solution of luminol?
anwer i got: molarity of luminol solution = 1.43m b)before investigating the scene, the technician must dilute the luminol solution to a concentration of 6.00×10−2 m. the diluted solution is then placed in a spray bottle for application on the desired surfaces.
i cannot get the correct answer for "c" have tried: 172ml,11.9ml, and 1.19*10^4. the only other possibility that i can come up with is: 83.9ml. would this one be i still completely out to
c)how many moles of luminol are present in 2.00 l of the diluted spray?
anwer i got: moles of luminol = 0.120mol what volume of the stock solution (part a) would contain the number of moles present in the diluted solution (part b)?
express your answer in milliliters.

Answers: 3
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 20:00, aksambo4707
Many free radicals combine to form molecules that do not contain any unpaired electrons. the driving force for the radical–radical combination reaction is the formation of a new electron‑pair bond. consider the chemical equation. n(g)+no(g)⟶nno(g) n(g)+no(g)⟶nno(g) write lewis formulas for the reactant and product species in the chemical equation. include nonbonding electrons. n(g)n(g) select draw rings more erase select draw rings more erase select draw rings more erase n no(g)
Answers: 1

Chemistry, 23.06.2019 00:00, jasmin5285
What is the approximate mass of 25 cm3 of silver, if the density is 10.5 g/cm3? a. 0.42 g b. 2.4 g c. 42 g d. 260 g
Answers: 1
Do you know the correct answer?
On part "c": the forensic technician at a crime scene has just prepared a luminol stock solution by...
Questions in other subjects:

Social Studies, 18.10.2020 14:01

Mathematics, 18.10.2020 14:01






English, 18.10.2020 14:01

Mathematics, 18.10.2020 14:01

English, 18.10.2020 14:01